The FIXION® Knee Ligamentoplasty System consists of PDLLA Copolymer Bioabsorbable Interference Screws (70% L Lactic Acid, 30% D Lactic Acid), TA6V Femoral Buttons with a fixed or adjustable HS Fiber® loop, and instrumentation for Kenneth-Jones or Hamstring-type ligamentoplasties.

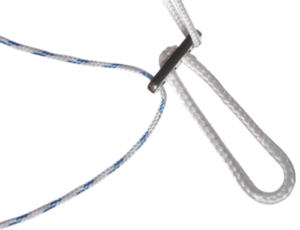
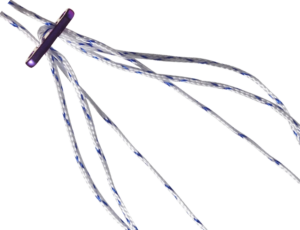
The FIXION® implants belong to the class III implantable medical device classification. The FIXION® implants are indicated for fixing the autologous graft in the LCA ligamentoplasties of the knee.
The surgeon is required to read the instructions for use (IFU) leaflet S12 0306 included in the packaging of the implant or available for download on the www.evolutisfrance.com website, as well as the surgical technique manual (G26 451) initially delivered with the instrument set, or equally available for download on the www.evolutisfrance.com website.
HEAD OFFICE
PRODUCTION SITE
10 place des Tuiliers
42720 Briennon
France
LOGISTIC HEADQUARTER
18 avenue de la Libération
42720 Briennon
France
© 2020 Evolutis – Production www.oz-media.com
You must be logged in to view these documents